1. Project Title
New functionalised materials for removal of pollutants from drinking and wastewaters
2. Participants
Kovalchuk Tetyana / Doctor, Researcher at the Department of Analytical Chemistry
B. Host Institution Director
Zaitsev Volodymyr/ Professor, Chief of the Department of Analytical Chemistry
3. Scientific Report
3-1. Short overview of the scientific activity undertaken
As a main strategy of this project, we planned developing cheap and easily available adsorbents based on natural materials. Groundwork for this direction was started during the first project year, by choosing convenient natural Ukrainian materials. During the second year of the research, this direction was thoroughly explored and the prospective of the application of natural Ukrainian clays for adsorbent make was evaluated.
Materials studied are natural silica – TREPEL, non-swelling clay-PALYGORSKITE and swelling clays MONTMORILLONITE and BENTONITE (1-6). The scientific activity carried out involved ACTIVATION; CREATION OF THE ACTIVE SITES ON MATERIALS SURFACE BY FUNCTIONALISATION WITH AMINOSILANES; and THEIR SUBSEQUENT TRANSFORMATION INTO S,N- AND P,N-CONTAINING CHELATORS (milestone 2); CHEMICAL AND PHYSICAL CHARACTERIZATION OF THE MATERIALS (milestone 3), AND WAS MAINLY FOCUSED ON milestones 4 (ADSORPTION STUDIES).
The adsorbents designed for the use at centralised water purification stations and those supplied in home filters target cheap and, desirably, selective materials which are subject to regeneration. To meet the criteria of cost-effectiveness, use of low cost natural and renewable adsorbents is mostly potential. Natural minerals are cheap and abundant solids. Due to their local availability, technical feasibility, engineering applications and cost effectiveness, the use of clays for waste and drinking water treatment can be recommended. However, natural materials generally lack binding strength and selectivity with an exception of certain laminar minerals which are excellent ion-exchangers on their own right.
Bibliographic analysis shows that a number of surface modifications ranging from acid and thermal treatment, to pillaring with organic cations and furthermore, to sophisticated grafting of silanes can be done to clays. They can be rendered selective adsorptive properties if tailored with chelating functions. While functionalisation of synthetic silica with chelating functions has been a subject of numerous studies, functionalisation of natural silicas and natural clays received little attention, and a vast potential lays within their functionalisation and selectivity improvement. We suggest using clays, non-selective ion-exchanger materials, and tailor their adsorption capacity and selectivity for removal of hazardous metal ions from drinking water via functionalized with chelating groups.
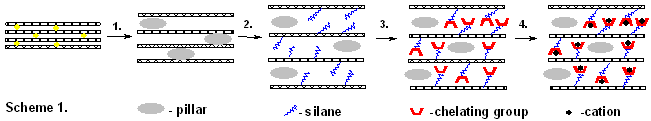
As it follows from the above-said, the main scientific objective of our study consisted in developing a SYNTHESIS METHODOLOGY for improving adsorptive properties (selectivity and capacity) of clays (Scheme 1). This included sub-objectives:
o To study how structural parameters (basal distance, surface area, hydrophobicity) of clays vary with modification procedures applied (activation with solvents, acids, thermal treatment , pillaring with inorganic pillars (Stage 1, Scheme 1), silanes and others);
o To optimize the procedure of intercalation/or grafting of various types of functional silanes (Stage 2, Scheme 1). Elucidation of the mechanism of silane-clay interaction, and of an exact grafting location;
o Tailored transformation of grafted silane molecules into chelating ligands to improve selectivity to mentioned cations (Stage 3, Scheme 1). Amongst the most useful P,N,S-containing complexons we prepared derivatives of ethylenediamines, diethylentriammines, aminophosphonic acids and sulphur-containing ligands, such as thiols and thioureas.
o Study of the adsorptional properties of modified/functionalised clays as compared to natural clays. Correlations between the structure of chelating group, the hydrophobic properties of the material and the selectivity/capacity of adsorption of toxic ions (Stage 4, Scheme 1).
o

3-2. Short description of the most important scientific results and achievements
3-2-1. Trepel. Functionalisation. Adsorption properties. Perspectives
CHARACTERISTICS Trepel (denoted in what follows as Tp) is a light porous, slightly cemented siliceous mineral, formed by round-shaped particles of silica, with the diameter 1000-2000 nm, density of 0.5 – 0.8 g m-3, and porosity of around 60 – 70%. According to X-Ray diffraction study, the studied sample is low-crystalline and consists of a mixture of Tridymite and Quartz mineral. According to N2 adsorption, Tp is characterized by relatively low surface area (S=56 m2 g-1).
|
|
|
|
|
|
|
Figure SEQ Figure \* ARABIC 1. X-Ray Diffraction pattern of starting Trepel |
|
|
|
FUNCTIONALISATION Initial Tp consists of big particulate, and according to pH study, conatins strongly basic impurities (Figure 2, curve 1: pH of suspension is 9, the inflection point at pH 5). Therefore, before functionalisation, Tp is activated by grinding and acid wash. Activated Tp is purely siliceous, as shows absence of species of basic and acidic character during its titration (Figure 2, curve 3); and presence of Q4, Q3 and Q2 species in its 29Si MAS NMR spectrum (Figure 4). Considering siliceous nature of Tp (silanols as active surface sites), its functionalisation was performed via covalent grafting of functional silanes in anhydrous conditions. Activation and silanisation procedures used are described below.
|
|||
Activation. Trepel is ground and fractioned by sieving. 20 g of Tp is activated through hydroxylation via reflux in HNO3 (10%, 100 ml) for 12 h and washed with water until the complete removal of Н+. Before silanisation, the sample is dehydrated and partially dehydroxylated by calcinations at 200-250С for 5 h and cooled in a closed dessicator. Silanisation. Activated Tp (10 g) is placed in a trhree-neck with 20-30 ml of a solvent (toluene, or dioxane). To this suspension the solution of silane (aminopropyl-, ethylenediaminopropyl-, diethylenetriaminopropyl triethoxysilane), 1 mmol/g, Et3N or Py (1 mmol/g) in 5 ml of solvent is added. The suspension is heated at 100 C under constant stiring for 10-12h. Washing and drying. Sample is washed in Soxlet with a boiling solvent (non-polar followed with a polar one) for 10 h to remove non-grafted silane (controlled by bindone). Sample is air-dried overnight, and then calcined at 150-180 С for 12 h in vacuum to afford condensation of unreacted silane. d) Hydration and improvement of hydrolytic stability To ensure hydrolityc stability of grafted layer and constant adsorption performance of samples, after silanisation and calcination the samples are hydrated by dispersing in a distilled water for 24 h, then separated by filtration, thoroughly washed and air-dried first at 25C followed by calcinations at 120-140С for 12 h.
CHARACTERISTICS OF ADSORBENTS Adorbents prepared based on functionalized Tp are shown on Scheme 2. Concentration of the grafted aminofunctions was determined from the potentiometric titration (Figure 3). By functionalisation we achieved relatively small concentrations (С Li per g) due to a relatively small surface area of starting Tp. At the same time, high density is achieved: high С Li per nm2 [0.6-1.5 groups per nm2]. This suggests submonolayer coverage of surface [СOH 1.8-2.3 groups per nm2] with silane. X-Ray diffraction shows identity of the crystallinity of support and functionalised samples. Solid state MAS NMR spectra of grafted Tp-samples provide an evidence to the covalent bonding of silane to the surface of Tp. Comparison of the spectrum 1 with spectra 2 and 3 (Figure 4) shows significant decrease of the relative intensity of the peak due to Q3 species (Scheme 3: silanol groups) as compared to the spectrum of initial Tp-sample.
|
|
|
|
|
|
|
Scheme 2. |
Scheme 3. |
|
|
|
|
|
|
|
Figure SEQ Figure \* ARABIC 3. Potentiometric titration of functionalised Тр (m=0.2 g, V =20 ml of 0.1 N NaCl) with 0.1N HCl. |
Figure SEQ Figure \* ARABIC 4. |